In The Current State of Drug Price Transparency Reporting webinar that recently aired, Rupal Patel, Senior Director of Advisory Services, and John Whitridge, Manager of Advisory Services, discussed a growing concern with pharmaceutical manufacturers: State Pricing Transparency Reporting (SPTR). Here is a breakdown of what was discussed, how it complicates the regulatory landscape, and what manufacturers can do to mitigate risk.
What is State Pricing Transparency Reporting?
Over the past few years, almost half of all states have signed, or are in the process of signing into law pharmaceutical pricing transparency measures which place new compliance and reporting obligations on manufacturers. These new regulations are designed to limit drug costs by requiring manufacturers to report on drug prices and price increases.
How did this begin?
State Pricing Transparency legislation is a response to the industry trend of increasing Wholesale Acquisition Cost (WAC) prices and the lack of public knowledge around the reasoning for those increases. Individual states have taken it upon themselves to take legislative action in order to raise awareness for drug pricing trends over time allowing more accurate cost forecasts for state budgets.
What challenges do manufacturers face?
State Pricing Transparency Reporting presents a set of challenges to manufacturers, such as:
- The need to establish a baseline understanding of the complex landscape of state-by-state, expanding regulations and must proactively monitor new pricing transparency legislation.
- The need for a business partner who can provide compliance and strategic consulting support related to compliance with applicable pricing transparency laws.
- Significant increases to administrative and operational bandwidth as the requirements and timing of reports are not the same from state to state.
- The overall cost of compliance to the manufacturer (annual fees for state registrations).
What does the current SPTR landscape look like?
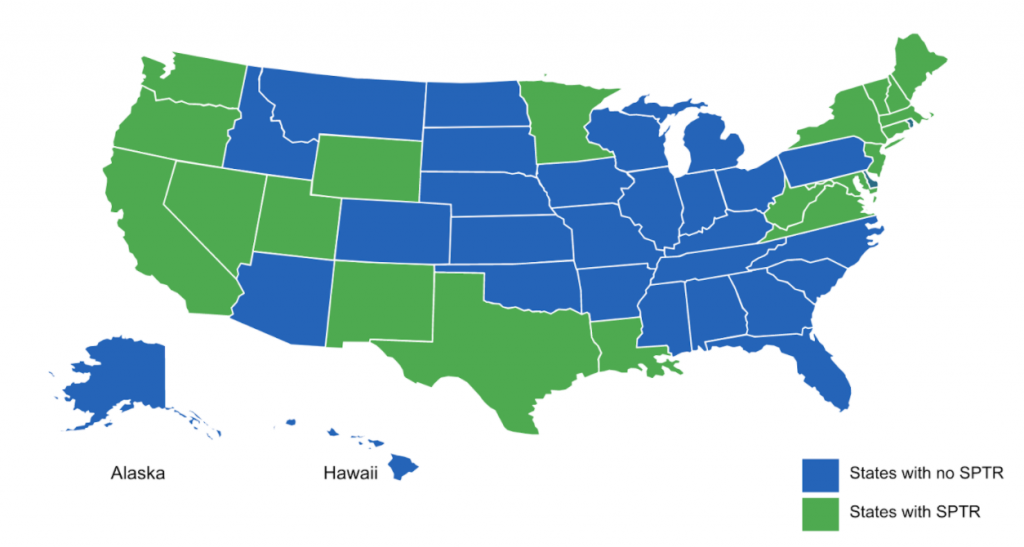
This is a snapshot of states that as of 2021, have enacted or expanded state price transparency laws in addition to current state legislation
What does the future of SPTR legislation hold?
It’s likely that this type of legislation will continue as more states seek to control the cost of pharmaceuticals in their annual budget. The Biden administration has indicated that they support the public knowledge of drug pricing and there is the potential for federal-level policies to complement state regulations.
How can IntegriChain help?
When speaking with a current IntegriChain customer, an SVP of Market Access at a mid-market pharmaceutical manufacturer, he had this to say about his decision to use IntegriChain’s state pricing transparency reporting:
“We were getting a patchwork in regards to state price reporting requirements and other state obligations. There are 50 states, all with different reporting requirements and there’s only one of me. It’s a lot to keep up with since it’s a constantly evolving area. Since it’s so much to manage, I believe it is frequently overlooked within the market access world, but it can really hurt you if you’re not paying attention to it.”
The first thing to know is that you don’t have to go it alone. IntegriChain is a trusted partner to over 60 pharmaceutical manufacturers, and can help with:
- Pricing transparency law trackers
- Communication and training tools
- Ongoing monitoring services
- Pricing calculator
- Reporting capabilities
In addition, IntegriChain can also communicate with state agencies. Since many bills have vague language, being able to confirm unique contracting or manufacturing scenarios directly with state agencies can save your organization time and trouble.
And most importantly, our subject matter experts know that constant monitoring and awareness is key, since:
- States may adjust reporting timelines forward or backward when releasing new material
- Automating the monitoring and timeline process avoids the potential for incorrect reporting dates
- Dedicating time to clean up efforts when beginning the project helps the rest of the project run significantly smoother (quicker time to steady-state for onboarding customers)
If you’re interested in achieving operational readiness and reducing compliance risk, reach out to price.transparency@integrichain.com.
Want to learn more?
Click here to hear a full replay of this webinar.
Interested in other government program adherence? Learn more about reducing revenue leakage with a better Medicaid Claims Level Data (CLD) process.







