The term rare disease is somewhat misleading for casual observers of the biopharmaceutical industry. Conditions considered “rare” are actually quite common and new drug approvals have recently skewed in their direction. Rare diseases affect 30 million people in the United States, which roughly equates to 10% of the population. In 2017, 40% of the novel drugs approved by the FDA were designated to treat a rare disease.
As approvals for rare disease treatments increase, these products account for an ever increasing share of overall pharmaceutical sales. In 2017, rare disease therapy sales totaled $125B and the outlook for rare disease therapies is strong going forward. In its Orphan Drug Report 2018, EvaluatePharma forecasts 2024 global orphan drugs sales of $262B with a CAGR 2018 to 2024 of +11.3% ⏤ approximately double the overall prescription market growth. By 2024, orphan drugs will account for an estimated 21.7% of worldwide prescription sales (excluding generics).
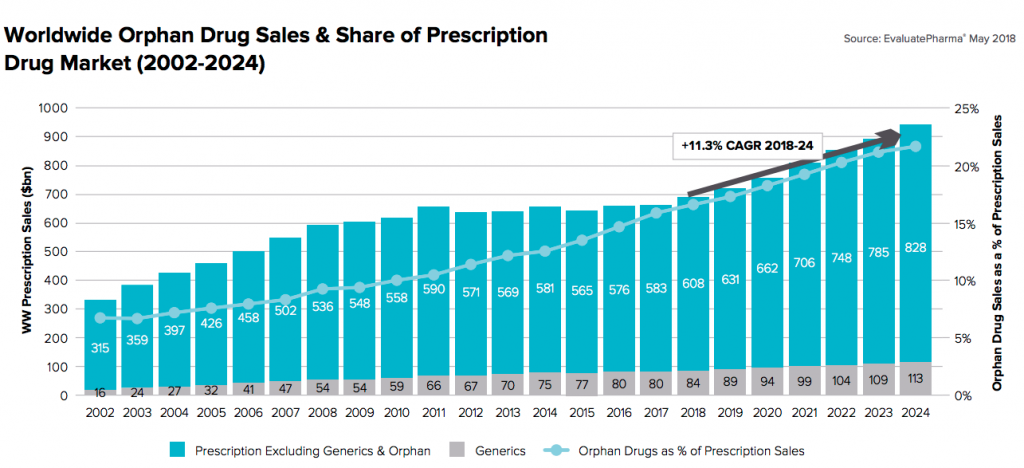
Figure 1: Growth of orphan drug prescription sales as a percentage of the overall market. Source – EvaluatePharma
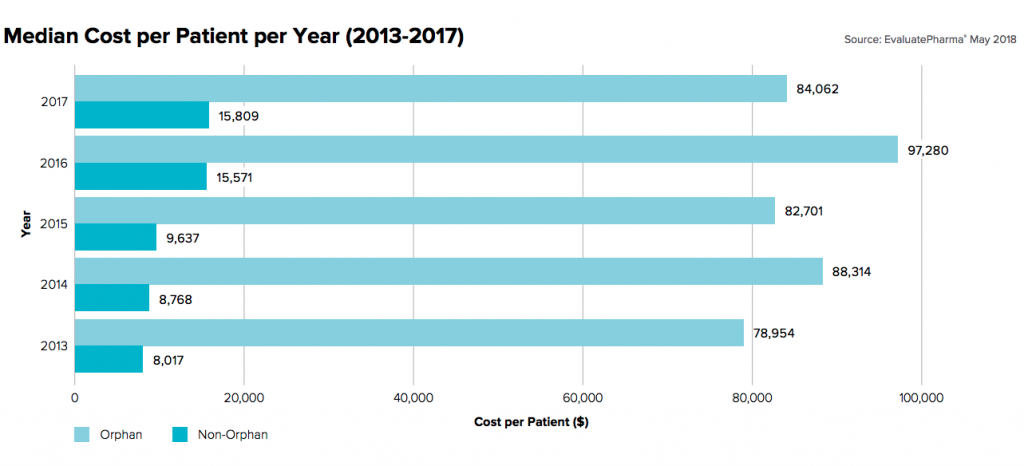
Figure 2: Annual per patient cost of orphan drug products. Source – EvaluatePharma
As pharmaceutical companies look to offset commercialization costs across smaller, more targeted patient populations, per patient costs are sharply increasing. In the eyes of Payers responsible for covering a large portion of treatments costs, this trend looks unsustainable in the long-term and is impacting commercial contract negotiations with manufacturers in the near term.
Payer Reaction and Effect on Contract Strategy
In recent discussions with Payers, it is clear the term rare disease has become synonymous with expensive. Looking to offload some of risk for covering costly rare disease treatments, Payers are asking manufacturers for meaningful data to demonstrate:
- The importance of the therapeutic area (by showing that the costs of therapy are backed by objective value cases)
- Efficacy of treatment measured post-launch
Prior to launch, quantifying value is the ideal. Hemlibra, a weekly injection indicated to reduce bleeding episodes in patients with hemophilia A, was one of the rare disease treatments approved by the FDA in 2017. A report issued by the Institute for Clinical and Economic Review (ICER) found that Hemlibra is cost effective despite its price tag of $482,000 for the first year and $448,00 for subsequent years. The agency’s report found that Hemlibra would reduce the overall U.S. healthcare budget by $720,000 per patient annually for children under 12. The healthcare system saves an even greater $1.85 million annually for persons over the age of 12.
Hemophilia A happens to be a competitive therapeutic class with the benefit of comparative products.First-in-class therapies (backed by single arm clinical trials) lack an active comparator and by definition do not allow for an accepted ICER (incremental cost effectiveness ratio) study. In situations where demonstrating a quantifiable value case is not an option for innovative brands, deal models that require both parties to share risk can be extremely helpful.
Value-Based Contracting for Rare Disease Products
In response to the Payer community’s reach for value, manufacturers may benefit from employing either value-based or outcomes-based contracting. When dealing with high cost therapies, the Payer community has a meaningful desire for manufacturers to have more skin in the game tied to results, especially where comparator data is sparse. At the same time, manufacturers are incentivized to have as few restrictions as possible between the patient and their therapy. This shared objective of a value-for-access incentive structure creates a clear benefit for all stakeholders to engage in risk-sharing agreements.
Meaningful analytics have a role to play in forecasting the potential bottom-line impact and degree of risk embedded in these innovative, value-driven deal structures. For example, manufacturers should leverage clinical data to forecast their exposure to financial liability tied to endpoints in value-based agreements. Data-driven deal execution is also helpful when constructing out clauses in value-based agreements where discounts would no longer apply.
The importance of sound analytical modeling and a more rigorous legal review is even greater with innovative agreements given the heightened risk of an unintended consequence. The added effort can be well worth it for all parties involved when value is well aligned.
About the Author
David has dedicated his career to delivering strategic and business process solutions to his pharmaceutical and biotechnology customers, primarily in the areas of managed markets strategy, contract analysis, and fact-based negotiation. He has consulted for many of the top pharmaceutical and biotechnology companies on issues ranging from merger integrations, pharmacoeconomics, and major product launches. David serves as IntegriChain’s Segment Leader for Payer and Market Access.









